Abstract
Introduction: Monoclonal P-selectin blocking antibody (Crizanlizumab, ADAKVEO®, Novartis, Switzerland) targets reduction of vaso-occlusive crises (VOCs) in adult and pediatric patients (≥16 years) with sickle cell disease (SCD). Patients with SCD have been reported with endothelial p-selectin to be initiatory for endothelial adhesion of sickle red blood cells (RBCs). Herein, we report the novel endothelium-on-a-chip (microfluidic platform - MFP) that enables assessment of Crizanlizumab on the adhesion of RBCs to perfusion-cultured human endothelial cells (ECs) in acute and chronic activation setup.
Methods: Whole blood samples collected from SCD subjects (n=14), HbSS and in EDTA and sodium citrate. RBCs were isolated via centrifugation from whole blood and resuspended in basal cell culture medium (EBM; Lonza, Morristown, NJ, USA) at a hematocrit of 20% with 10 mM of HEPES. Human umbilical vein endothelial cells (HUVECs; Lonza, Morristown, NJ, USA) were cultured within the microfluidic platform channels at 15 dyne/cm2 for at least 48 hours prior to experiments. To mimic the acute EC activation, blood samples were supplemented with 40 µM heme +/- 100 µg/ml Crizanlizumab and injected through the microfluidic channels for 15 minutes. Instead to mimic chronic pre-activation in SCD HUVECs were pretreated for 4 hours with TNFα (20 ng/ml) or 50% plasma of SCD patients in basal media before, +/- 100 µg/ml Crizanlizumab followed by injection of blood samples through the microfluidic channels. Non-adherent RBCs were removed via washing solution with or without Crizanlizumab and the remaining RBCs were imaged using an inverted microscope and the manual quantification was completed based on characteristics previous published. Paired t-test to evaluate the effects of the Crizanlizumab on RBCs adherent to activated EC has been used.
Results: We observed a statistically significant reduction of RBC adhesion due to Crizanlizumab treatment in 15-minutes acutely heme activated ECs (135±40 vs 1513±617, p≤0.05). The effect of Crizanlizumab on decreasing RBC adhesion was also statistically significant when ECs were chronically pre-activated by TNF-α followed by a 15-minute heme activation (4404±1393 vs 2016±609, p≤0.05). To further elaborate on the effects of Crizanlizumab in the atherogenic SCD population, we activated the EC with subjects' autologous plasma for 4 hours followed by a 15-minute heme activation, (5876±2579 vs 2397±1381p≤0.05).
Discussion and Conclusions: Per label use of Crizanlizumab to reduce VOC, application of novel MFP associated with Crizanlizumab use displayed a reduced RBC adhesion to acutely heme-activated EC. Effects were preserved in presence or absence of chronic TNFα or autologous plasma pre-activation. Endothelium-on-a-chip MFP has been shown as reliable in monitoring patient response to anti-adhesive therapies in SCD population.
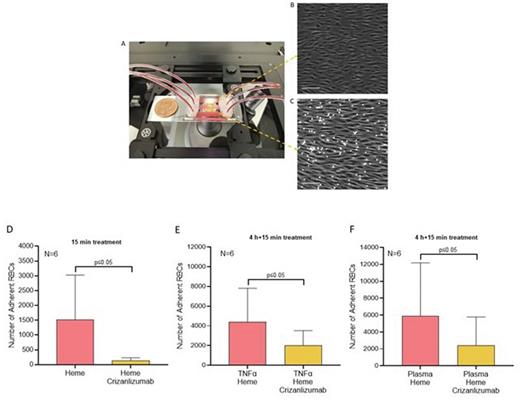
Fig 1. Endothelium-on-a-chip MFP set up (A). The phase-contrast image of HUVECs demonstrate a confluent layer of cells aligned with flow. (C) RBCs adhesion to activated HUVECs. Effects of Crizanlizumab on RBC adhesion levels to HUVECs activated for 15-minutes with heme (D); 4-hours with TNF-α and 15-minutes with heme (E); 4-hours with plasma and 15-minutes with heme(F). Microscope images at 10X. Scale bare 100um. Error bars represent the standard error of the mean (SEM).
Disclosures
Federici:Biochip Labs: Current Employment. Kucukal:Biochip Labs: Consultancy. Sertel:Biochip Labs: Current Employment. Combs:Biochip Labs: Current Employment. Bruederle:Novartis Pharma AG: Current Employment. Zak:Biochip Labs: Current Employment, Patents & Royalties. Gurkan:Dx Now Inc.: Patents & Royalties; Hemex Health, Inc.: Current Employment, Patents & Royalties; Biochip Labs: Patents & Royalties; Xatek Inc.: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.